Groundbreaking research
Unprecedented scale
Such large-scale genetic research into the origins of ALS is unprecedented! As such, we are fully committed to making a revolutionary breakthrough in the search for the cause of ALS. But, to reach our goals, we need your help.
No treatment yet
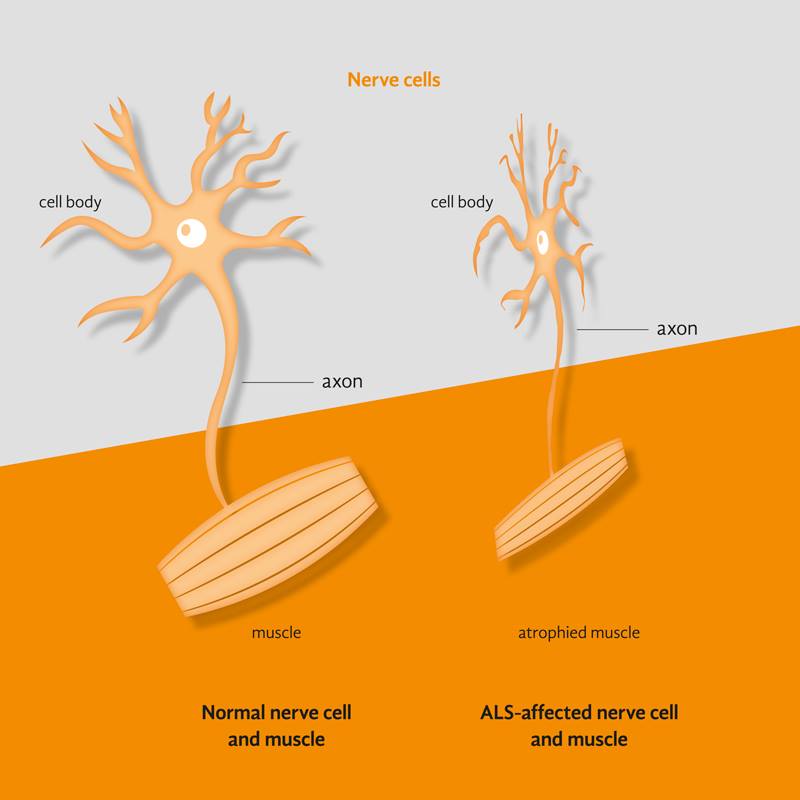
More than 200,000 people worldwide are living with Amyotrophic Lateral Sclerosis (ALS), otherwise known as Motor Neuron Disease (MND) or Lou Gehrig’s disease. Relatively little is known about the cause of this progressively degenerative neurological disease. There is still no treatment. The average life expectancy of ALS patients is three years.
Find a cure
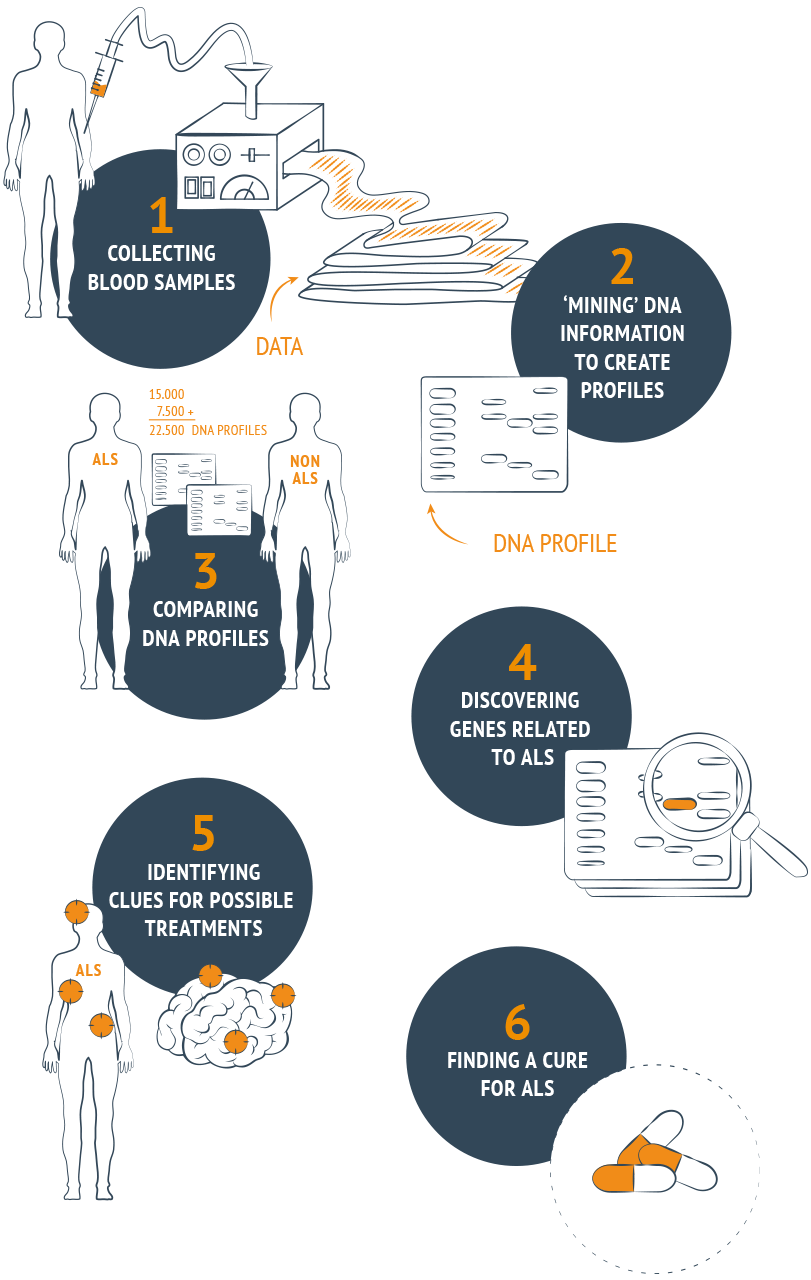
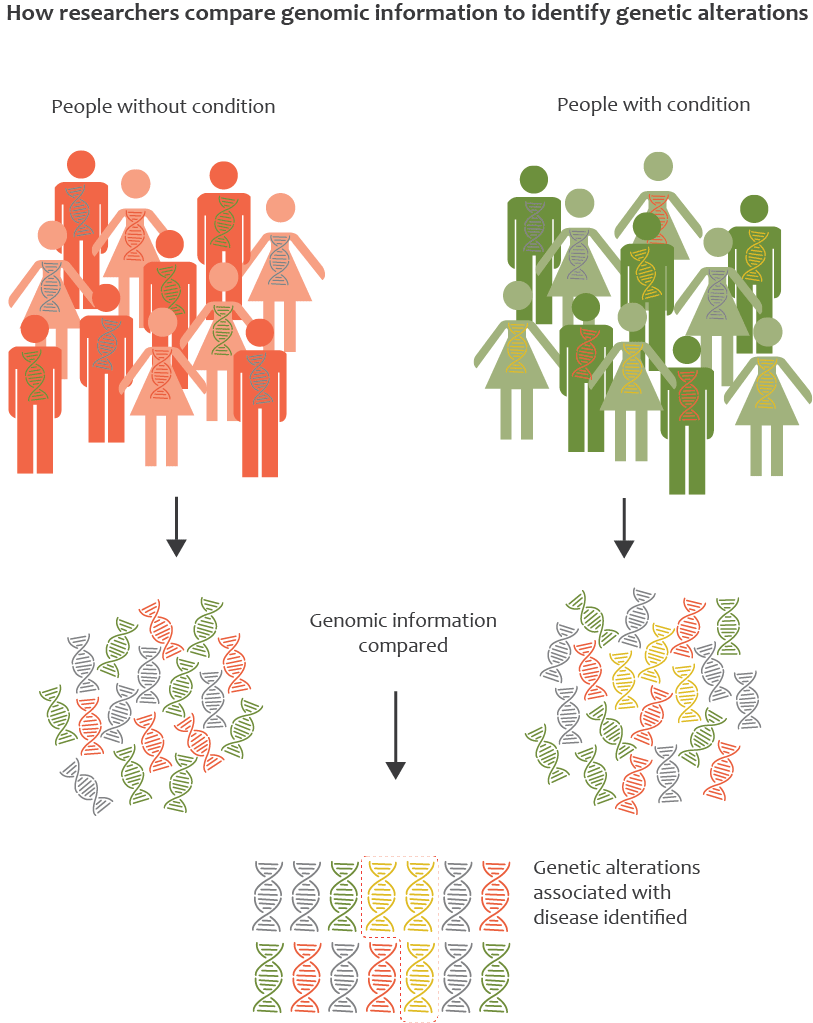
It is almost certain that the disease has a genetic basis. Project MinE is a large-scale research initiative devoted to discovering the genetic cause of ALS. The ultimate goal is to identify genes that are associated with ALS. The function of these genes may lead to disease pathways for which treatment can be developed.
Help us
In order to reach this ambitious objective, we plan to map the full DNA profiles of at least 15,000 people with ALS and compare them to DNA profiles of 7,500 control subjects to uncover associations between specific variations in genes and ALS. This type of comparative research requires enormous numbers of DNA profiles and is very costly. That is why we need your help. Make a donation or start a campaign today! Project MinE, make it yours!